My Interests
I am interested in understanding how species respond to environmental change. Certain environmental conditions are beneficial while others are harmful to animals. Trying to understand what species will be tolerant or intolerant to particular environmental conditions is crucial for effective species management and environmental regulations in the face of climate change.
I study a variety of animals from shellfish to sea lice to salmon. I use genomics technologies and bioinformatics tools to perform molecular surveys that measure molecular responses on a broad scale. This holistic approach is similar to how a mechanic might look under the hood of a car to examine how parts collectively influence the performance of the car. I pair data from molecular surveys with health observations to understand how exactly animals compensate for changes in the environment. I aim for my research to identify environmental factors that lead to stronger animal responses, and help prioritize the protection of particular species and environmental mitigation strategies.
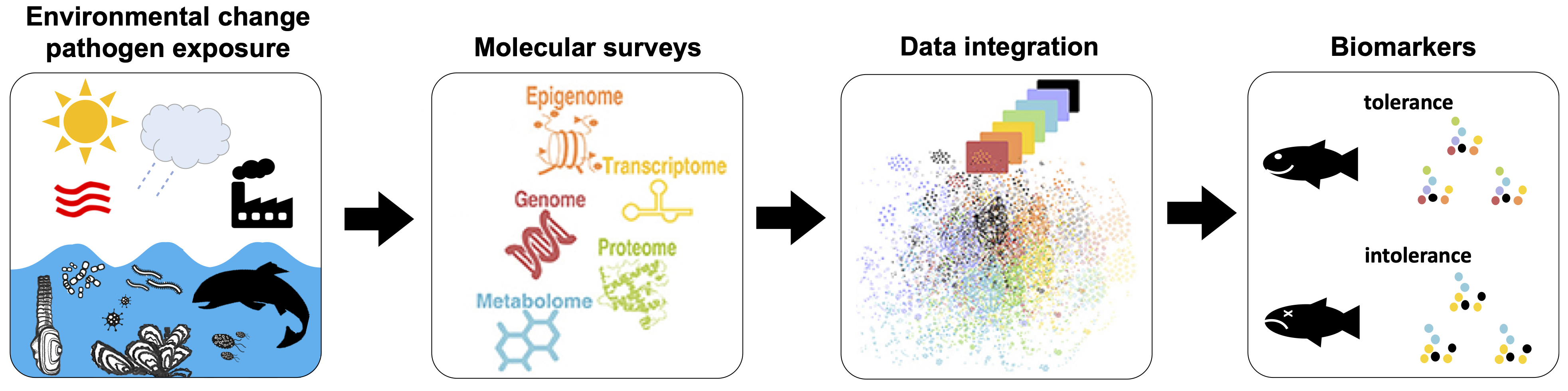 Schematic for identifying tolerance and intolerance signatures among marine animals experiencing physical and biological changes in their environment through integrating data from molecular surveys. Adapted from Vilne and Schunkert (2018) Front. Cardiovasc. https://doi.org/10.3389/fcvm.2018.00089.
Schematic for identifying tolerance and intolerance signatures among marine animals experiencing physical and biological changes in their environment through integrating data from molecular surveys. Adapted from Vilne and Schunkert (2018) Front. Cardiovasc. https://doi.org/10.3389/fcvm.2018.00089.
I am a proponent for open science and use an open access online lab notebook as well as GitHub to publicly share my research and support reproducibility.
I am currently a Research Scientitst at University of Washington School of Aquatic and Fishery Sciences and based in Gloucester, Massachusetts.
Current Research
Projects are listed chronologically starting with the most recent and resulting products can be found in the PublicationsDeveloping diagnostics for rapid detection of shellfish disease
Project summary can be found here section.Current methods for disease diagnostics in aquatic animals are typically expensive, complicated, and time consuming. There is a need to develop simple and economical field-deployable assays that deliver results rapidly, particularly in the aquaculture industry where early diagnosis of disease is critical to production. By adapting technologies that are currently being developed for the rapid detection of human diseases to aquatic animals, we can sensitively detect disease at an early stage to limit disease transmission and increase sustainability.
Temperature and salinity influence on sea lice-challenged Atlantic salmon
How does ocean warming and decreased salinity affect gene regulation in Atlantic salmon infested with sea lice?
Wild and farmed salmon are impacted by sea lice infestations leading to skin lesions, increased susceptibility to microbial pathogens, and up to a 10% loss in aquaculture production value. Particularly in Chile, farmed Atlantic salmon become infested by C. rogercresseyi. It is known that warmer temperatures can increase the sea lice generation time, and that higher salinity tends to lead to optimal development while lower salinity can impair larval development Towards developing improved methods for sea lice remediation, and in collaboration with Dr. Steven Roberts and Dr. Cristian Gallardo-Escarate (Universidad de Concepcion, Chile), I'm looking genome-wide for DNA methylation patterns in the skin of salmon infested with sea lice and exposed to decreased salinity, increased temperature, or a combination of the two. Differentially methylated regions among experimental and control groups indicate an epigenetic gene regulatory response driven by sea lice infestation and environment. These results provide insight into regulatory regions sensitive to methylation modification that underlie immune response to environment-specific sea lice infestation, and add clarity to genomic areas of vulnerability.
pH influence on Pacific geoduck development
Marine bivalve molluscs as calcifiers are susceptible to ocean acidification, but could some stress be good?
In collaboration with Dr. Steven Roberts and the Jamestown S'klallam Tribe's Fisheries division, I am investigating the effects of varying degrees of pH stress on broodstock reproductive development, and the performance of their offspring using a variety of molecular and physiological assays. To investigate epigenetic mechanisms of acclimatization to repeated low pH exposures within an early-stage juvenile populaiton, I'm examining genome-wide DNA methylation in collaboration with Dr. Hollie Putnam (URI). Early exposure led to compensatory growth and juvenile clams retained treatment-specific regions of differential methylation after 125 days being removed from the initial low pH treatment. Differentially methylated regions within specific genes and transposable elements shed light on putative functional roles of DNA methylation and mechanisms of intragenerational acclimatization. Taken together these data suggests that acclimatization to ocean acidification can result in benefits to geoduck growth, with exposure memory providing a mechanism for environmental hardening.
Combined low oxygen + low pH influence on pteropods
The planktonic pteropod is an important ecological species with roles in marine food webs, oceanic carbon cycling, and potentially serving as an indicator of ocean acidification. Although lab and field studies have shown pteropods are sensitive to ocean acidification conditions, in Puget Sound these organisms have been coping with low oxygen and aragonite saturation conditions during winters when food availability is low. To better understand the molecular mechanisms behind their resilience-yielding adaptive physiology, I'm interpreting the metabolomes and lipidomes of pteropods exposed to low oxygen and aragonite saturation conditions in collaboration with Drs. Krista Nichols, Shallin Busch and Paul McElhany of the NOAA Northwest Fishery Science Center. With low oxygen more strongly influencing both lipids and metabolites than aragonite saturation, preliminary biochemical pathway analysis suggests phosphatidylcholine metabolism is affected, which could be part of a mechanism for increasing oxygen transport. This study elucidates potential mechanisms of resilience to these environmental stressors and expands our overall understanding of how pteropods might respond to future ocean conditions.
Temperature influence on Pacific oyster development
The Pacific oyster undergoes a dramatic transformation from free-swimming larval form to sessile benthic dweller, and proliferates in environments widely ranging in temperature. In a hatchery setting, warmer seawater temperature during metamorphosis generally leads to higher survival. To investigate the molecular underpinnings of this observation, and in collaboration with Dr. Steven Roberts and Taylor Shellfish Hatchery, I applied a multi-statistical approach to characterize the proteomes of oysters at seven timepoints throughout metamorphosis at two different temperatures to better understand how developmental processes are altered. Over time, adhesion and calcification related proteins acutely decreased, organogenesis and extracellular matrix related proteins gradually decreased, proteins related to signalling showed sinusoidal abundance patterns, and proteins related to metabolic and growth processes gradually increased. Contrastingly, different sets of proteins showed temperature-dependent abundance patterns with proteins related to immune response showing lower abundance and catabolic pro-growth processes showing higher abundance in animals reared at a warmer temperature. These protein pattern differences correspond to larger oyster size observed at the elevated temperature and are likely indicative of a combination of differences in specific metamorphic processes and possible pathogen presence. The proteome resource generated by this study provides data-driven guidance for future work on developmental changes in molluscs.